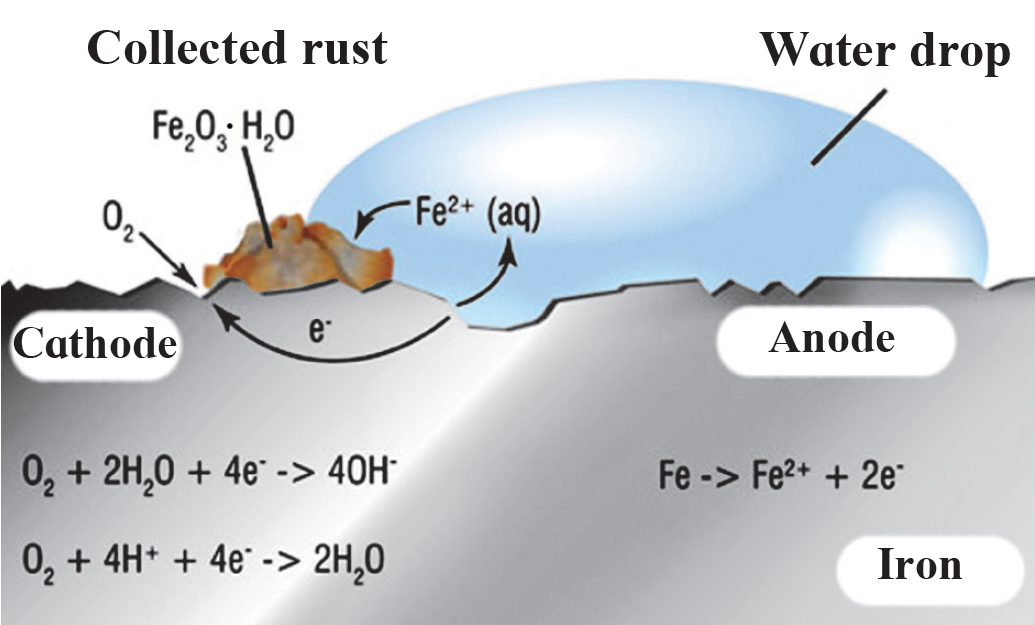
Observe the following picture a write down the chemical reaction with explanation:
 |
|---|
The chemical reaction shown here is rusting. Rusting is a complex electrochemical reaction. Different regions on the surface of iron become anode and cathode.
|